Inorganic Colloidal Dispersions
Metal oxide and noble metal nanoparticles are highly relevant for a variety of applications ranging from medical diagnostics to sun creams and from light harvesting quantum dots in solar cells to pigments in paints. In our group we focus on studies of particle formation, growth, aging, and functionalization. In any case the molecular structure of the solid-liquid interface between the nanoparticles and the dispersion medium is of particular interest and studied in situ by SAXS/SANS and several complementary methods. The interface structure is also studied at well defined planar metal oxide and nobel metal substrates, respectively, by XRR and GISAS.
Noble metal nanoparticles but especially gold nanoparticles (AuNPs) exhibit outstanding optical properties due to their plasmonic behavior. The absorption of UV- visible or NIR-light can be tuned by controlling size and shape of the AuNPs [1]. Gold nanorods (AuNRs) for example show two absorption bands due to their anisotropic shape [2]. To modify the morphology of AuNPs, surface-active substances like surfactants are needed to control formation, growth and stabilization of such particles. Cetyltrimethylammonium bromide (CTAB) is one of the most common surfactants and is used to prepare AuNRs in high yields and low polydispersity [3].
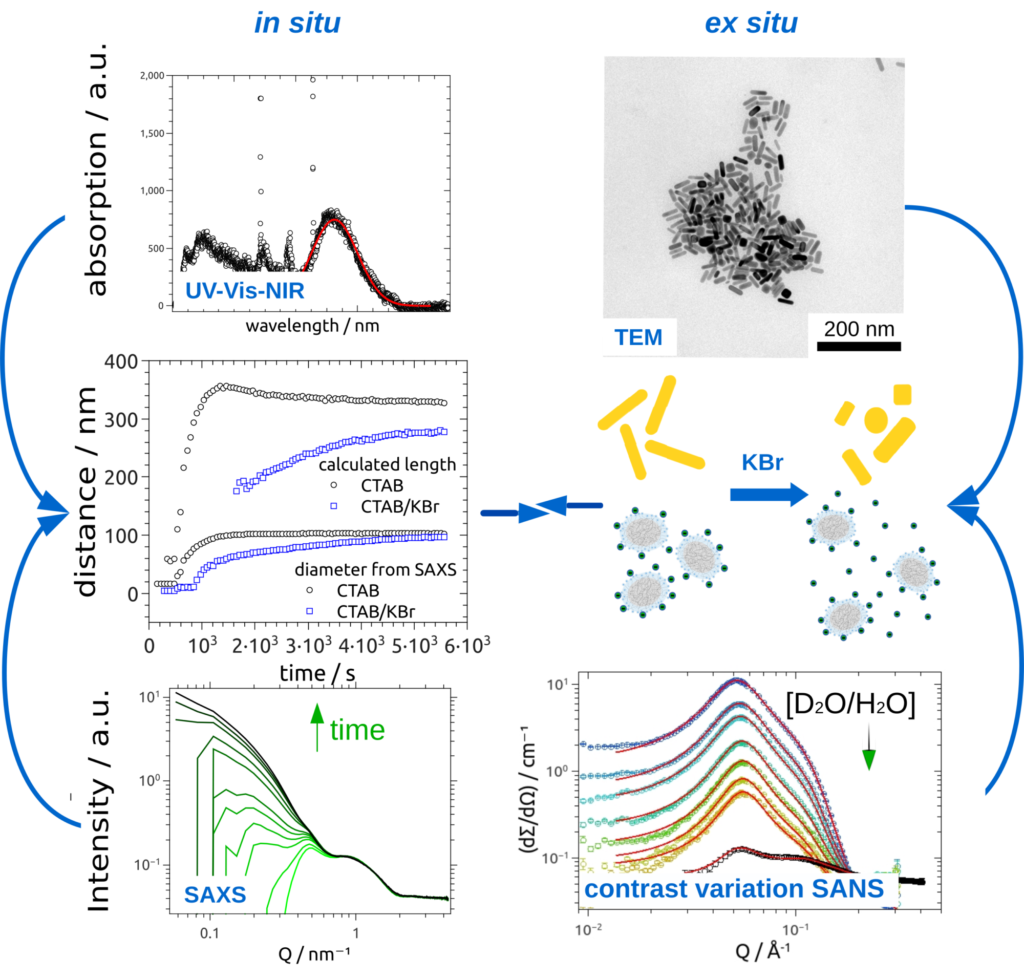
It is known, that CTAB forms micelles in aqueous solutions and a double layer to stabilize AuNRs [4]. We study the influence of CTAB micelles on the formation, stabilization and degradation of noble metal nanoparticles. Since the interactions between these particle systems are highly complex, a fundamental understanding of the underlying processes is still missing.
To characterize both noble metal nanoparticles and CTAB micelles, small angle scattering with X-ray (SAXS) and neutrons (SANS) is used.It is intended to contribute to a fundamental understand of the interaction between noble metal nanoparticles and micelles using a combination of in situ and ex situ SAXS, (contrast variation) SANS, UV-Vis-NIR spectroscopy, and transmission electron microscopy (TEM) studies (Fig.1).
[1] J. Pérez-Juste, I. Pastoriza-Santos, L.M. Liz-Marzán, P. Mulvaney, Coord. Chem. Rev. 249 (2005) 1870-1901
[2] C.J. Orendorff, T.K. Sau, C. J. Murphy, Small 2 (2006) 636-639
[3] N.R. Jana, L. Gearheart, C.J. Murphy, Adv. Mater. 13 (2001) 1389-1393
[4] S. Gómez-Grana, F. Hubert, F. Testard, A. Guerrero-Martinez, I. Grillo, L.M. Liz-Marzán, O. Spalla, Langmuir 28 (2012) 1453-1459
Growth of Metal Oxide Nanoparticles
We study the growth and ripening of ZnO and TiO2 semiconductor nanoparticles (NPs). These exhibit promising electro-optical properties which can be adjusted for different applications due to the quantum size effect. Thus, they can be integrated into electronic devices as well as in thin film solar cells where they act as an electron transfer system. For these applications stable and well-defined NPs have to be designed, and knowledge about the nucleation and growth processes as well as the stabilization is crucial.
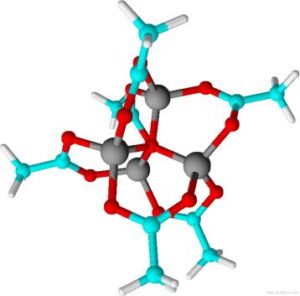
To synthesize ZnO nanoparticles we use a standard ansatz developed by Meulenkamp and Spanhel in which an ethanolic solution of zinc acetate dehydrate is heated for 3 h under solvothermal conditions to form a tetrahedral precursor (cf. Fig. 1). These precursor molecules consist of a tetragonal Zn4O unit and are stabilized by six surrounding acetate molecules. The addition of an ethanolic solution of the strong base LiOH to the precursor solution starts the ZnO formation.
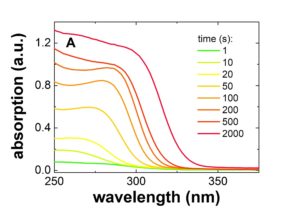
The formed ZnO NPs ripen in dependence of time and temperature to larger sizes. We study this ripening process by in-situ small angle X-ray scattering (SAXS) and absorption spectroscopy (UV-Vis) simultaneously. Typical time resolved SAXS and UV-Vis measurements of freshly prepared ZnO nanoparticles are displayed in figure 2. From these measurements the temporal evolution of the size and shape distributions of the nanoparticles can be determined and allow to get a detailed understanding of the kinetics of the underlying formation, growth and aging processes.
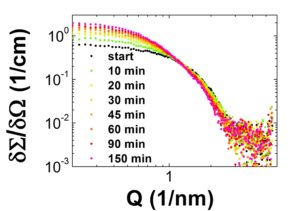
In addition to this well established sample system we recently started to study the influence of different bases on the synthesis of the ZnO nanoparticles as well as the effect of the addition of different stabilizer agents to the solution. Due to the compexity of these systems our SAXS studies need to be complemented by further techniques like small angle neutron scattering (SANS) which is in contrast to SAXS highly sensitive to the organic stabilizer molecules also in presence of the inorganic nanoparticles. To get insight into the native solution we also use cryo –TEM techniques.